Which of the Following Has the Lowest Ionization Energy
Down in a group ionization potential decreases so the ionization potential of sulphur is lower than the oxygen. This requires high amount of energy.
8 2 Periodic Trends Physical Science
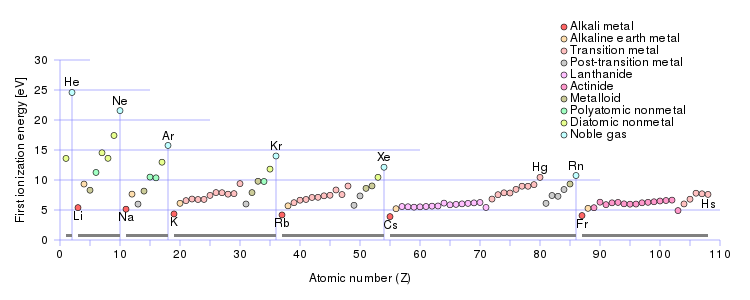
From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to.
. The electron configuration of the ions will match that of a noble gas. Expert answered alvinpnglnn Points 12475. The attraction of the nucleus for the electron becomes less and it becomes easier to pull it away.
Rank from highest to lowest ionization energy. This is because the electron to be removed from the outer energy level is increasingly distant from the nucleus as a result of the atoms getting bigger. So the decreasing order of ionization potential of all four elements is as follows.
On moving down the group the number of inner shells increase the ionization energy decrease the lowest ionisation energy is that of sulphur. Which type of semiconductor is created by doping with atoms that contain more valence electron than the semiconductor material. Cl P Si Mg Na.
The role does silver play in this chemical process is. Which of the following elements would have the lowest first ionization energy. In the Periodic Table the minimum ionisation energy is found at alkali metals.
What group has the lowest ionization energy. Also the electronic configuration of sulphur is N e 3 s 2 3 p 4. For example oxygen has a slightly lower ionization energy than nitrogen because nitrogen would lose half-full p orbitals while oxygen would gain half-full p orbitals.
Log in for more information. Which group has the lowest ionization energy. Which of the following elements has the lowest ionization energy.
Which of the following has lowest ionisation energy. Sulphur has the maximum atomic radius when compared to the other three atoms. Correct option is E The first ionisation energy decreases on going down a group.
Beryllium has the lowest ionization energy. A reaction between ethene and oxygen is used to produce epoxyethane. From the given elements Rb or Rubidium has the lowest ionization energy as it has lowest shielding effect so it easy to remove electron from its shell.
Silver is an n-type semiconductor in this reaction. Nitrogen Fluorine Oxygen Sulphur. The enhanced stability of this orbital symmetry is responsible for the deviation from the trend for these two elements.
The group of elements which have. Which of the following is the. Expert answeredAutaPoints 254 User.
For chemistry students and teachers. When silver is combined with ethene the reaction for producing epoxyethane occurs much faster. Consider the given options.
Asked 77 days ago252022 114006 PM. 119 rows - Ionization energy. Explain why Group 1 elements tend to form 1 ions and Group 7 elements tend to form 1- ions.
Log in for more information. From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon. From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon.
Score 1. So sulphur has the lowest ionization potential among all four. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group.
Which of the following elements has the lowest ionization energy. From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon. Cesium has the lowest ionization energy.
When Na ions loses an electron this stable configuration of Ne is broken. Energy released when excited electrons return to lower energy levels produce. Which group has the largest second ionization energy.
Ionization energy is defined required energy to eliminate or remove an electron from an ion or atom. By losing one electron sulphur can attain the half filled state and hence achieve greater stability. Ionization energies e none of the above C.
Asked 24 days ago3282022 34405 PM. Updated 6 minutes 8 seconds ago4242022 22259 PM. Hence sodium electronic configuration 1s22s22p63s1 has the lowest ionisation energy.
All of the above b. 1 day agoCesium has the lowest ionization energy. Hence second ionization energy of sodium is largest.
Therefore option C sulphur is correct. Hence sulphur has the least ionization enthalpy in this case. Hence sodium electronic configuration 1 s 2 2 s 2 2 p 6 3 s 1 has the.
The group of elements which have the lowest ionization energy are the alkali metals.

Ionization Energy Definition Chart Periodic Table Trend

The Parts Of The Periodic Table
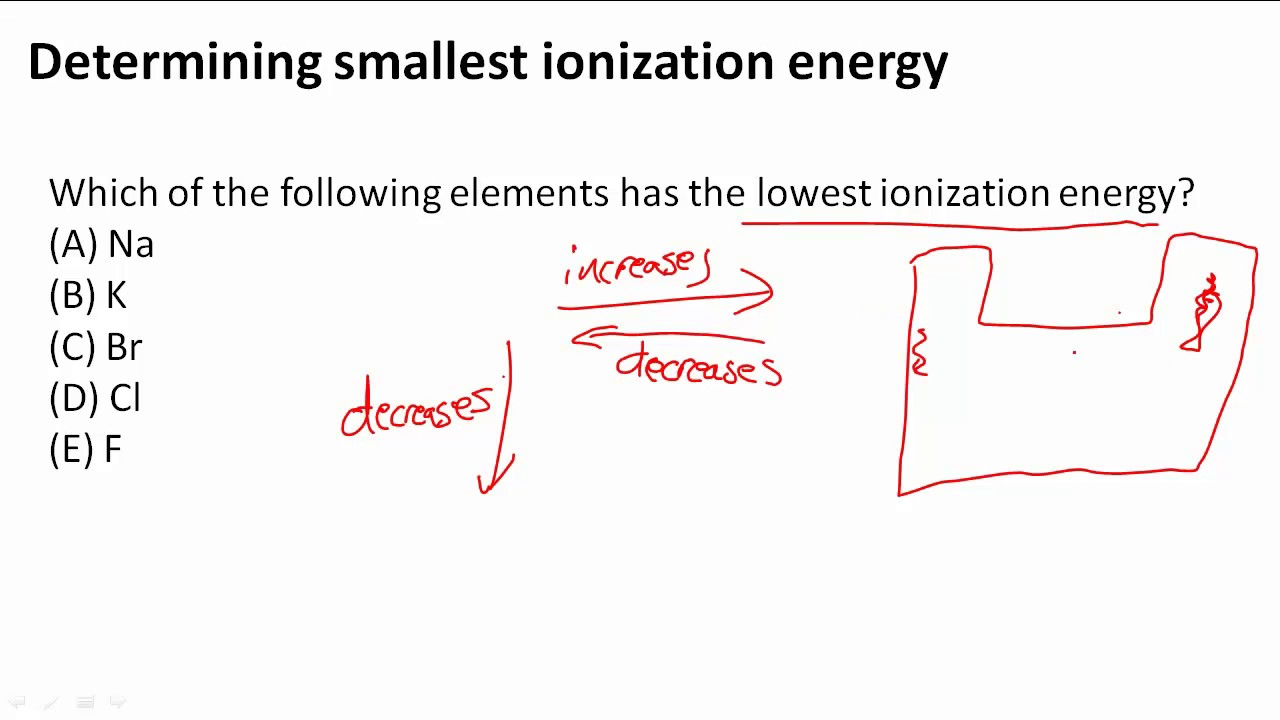
Determining Smallest Ionization Energy Youtube

Example Trend In Second Ionization Energy Youtube
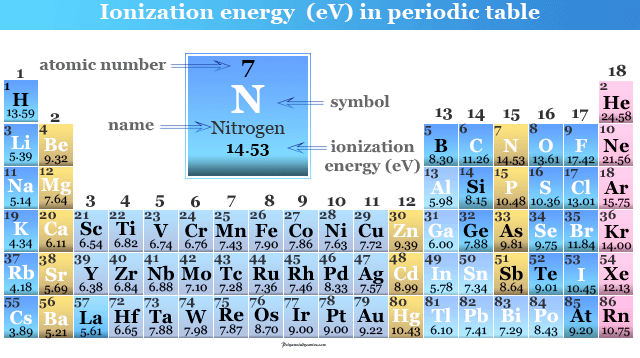
Ionization Energy Definition Equation Periodic Table Trends

Periodic Trends In Ionization Energy Ck 12 Foundation

What Family Has The Lowest Ionization Energy Socratic
What Is The Difference Between The Ionization Energy Of Na And Mg Quora

What Group Of Elements Generally Have The Lowest Second Ionization Energy

Solved Element Has The Lowest Ionization Energy
Ionization Energy And Electron Affinity

Inorganic Chemistry How Can I Relate The Reactivity Series To Electronegativity And Ionization Energy Chemistry Stack Exchange
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic

Ionization Energy Definition Chart Periodic Table Trend
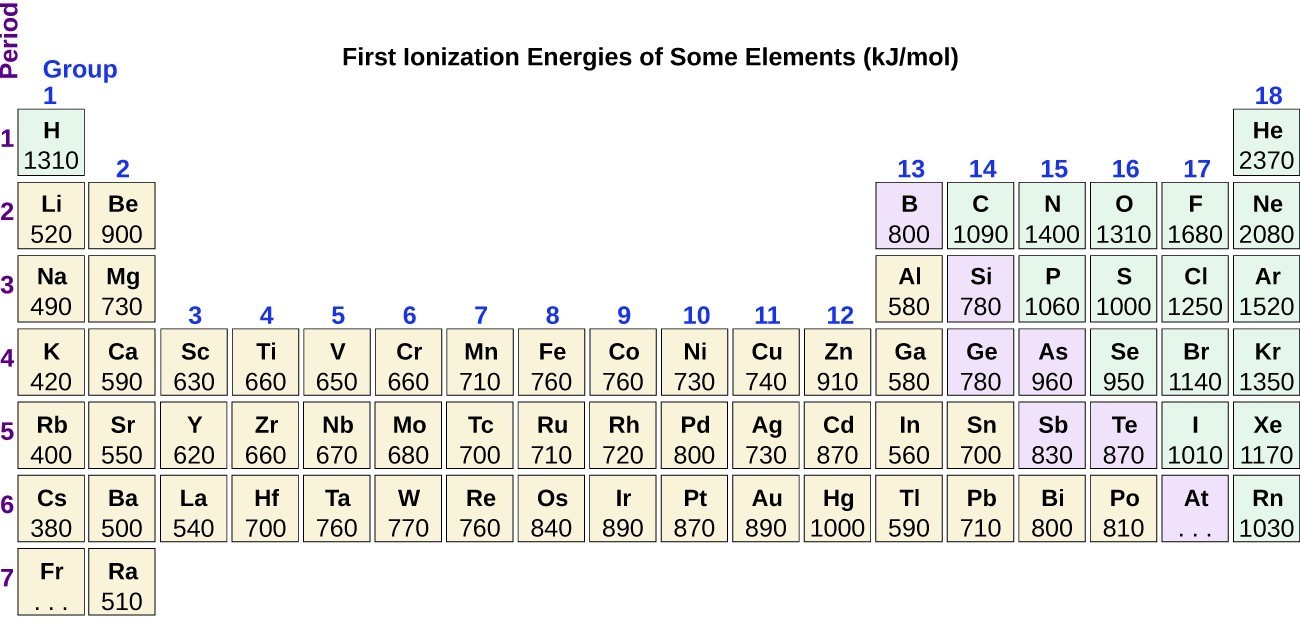
Periodic Variations In Element Properties Chem 1305 General Chemistry I Lecture
Ionization Energy Trends Of The Periodic Table

Which One Has The Lowest First Ionization Energy Ca P Or Ci Quora

Comments
Post a Comment